Answer: The amount of time needed is 4.53 minutes.
Step-by-step explanation:
The equation used to calculate time period follows:
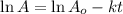
where,
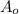 = initial mass of Cr-56 isotope = 34.5 mg
= initial mass of Cr-56 isotope = 34.5 mg
A = mass of the Cr-56 isotope left after the time = 20.3 mg
t = time = ? min
k = rate constant =
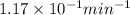
Putting values in above equation, we get:
![\ln (20.3)=\ln (34.5)-[(1.17* 10^(-1)min^(-1))* t]}\\\\t=(\ln(34.5)-\ln(20.3))/(1.17* 10^(-1))=4.53min](https://img.qammunity.org/2020/formulas/chemistry/college/q2sqyialc9ovig7xl4fs73acpwacd3f7dl.png)
Hence, the amount of time needed is 4.53 minutes.