Answer:
a. T and P remain the same (T=298 K and P=1 bar)
b. 11.23J/K
Step-by-step explanation:
a. Since the mixing process of an idea gas doesn't present a change in the enthalpy, we could state that no change in neither temperature and pressure are given.
b. It is not necessary to know enthalpy data, the following formula is enough to compute the entropy change:
Δ
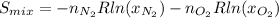
Thus, the molar fractions are equal to 0.5, and the result yields:
Δ
![S_(mix)=-(1mol)[(8.314J/(mol*K)]ln(0.5)-(1mol)[(8.314J/(mol*K)]ln(0.5)](https://img.qammunity.org/2020/formulas/chemistry/college/tf26xxfosrnydbm7ws5uqwnyy9y28meuaa.png)
Δ
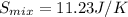
Best regards