Answer:
Least electronegative : Cs
Greatest nonmetallic character = Cl
Most likely to participate in bonding = Cs
Metalloid = Sb
Step-by-step explanation:
a) Electronegativity of the elements decreases down the group. Cs is placed in the bottom of the periodic table and have least electronegativity among given.
This can also be verified by electronegative values of the given element
Elecetonegativity of Be = 1.57
Elecetonegativity of C = 2.55
Elecetonegativity of Cl = 3.16
Elecetonegativity of Cs = 0.79
Elecetonegativity of Sb = 2.05
Hence, Cs is least electronegative among given.
b) Nonmetallic character increases across the period.
Cl is placed at the extreme right in the periodic table. So Cl has the greatest nonmetallic character among given.
c) Cs is most likely to participate in bonding.
Electronic configuration of Cs =
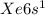
This is because, Cesium loses an electron and attains noble gas configuration. Cesium is the most reactive nonmetal and it even reacts with cold water.
d) Sb is a metalloid as it has both metallic and nonmetallic character.