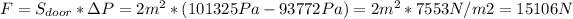Answer:
(a) Density of the air = 1.204 kg/m3
(b) Pressure = 93772 Pa or 0.703 mmHg
(c) Force needed to open the door = 15106 N
Step-by-step explanation:
(a) The density of the air at 22 deg C and 1 atm can be calculated using the Ideal Gas Law:
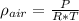
First, we change the units of P to Pa and T to deg K:
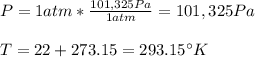
Then we have
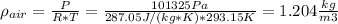
(b) To calculate the change in pressure, we use again the Ideal Gas law, expressed in another way:
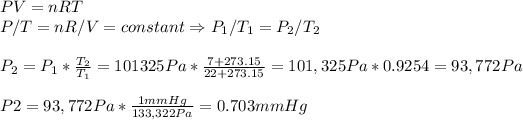
(c) To calculate the force needed to open we have to multiply the difference of pressure between the inside of the freezer and the outside and the surface of the door. We also take into account that Pa = N/m2.