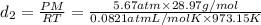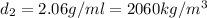Answer:
The air density in at points 1 is
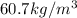 and 2 is
and 2 is
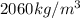 .
.
Step-by-step explanation:
Average molecular weight of an air ,M= 28.97 g/mol

or
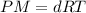
P = Pressure of the gas
n = moles of gas
T = Temperature of the gas
d = Density of the gas
M = molar mass of the gas
R = universal gas constant
Density at point-1 =
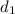

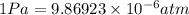
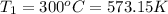
M = 28.97 g/mol
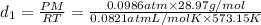
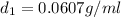
1 g = 0.001 kg
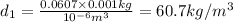
Density at point-2 =
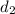
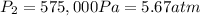
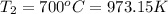
M = 28.97 g/mol