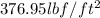Answer :
(a) The 60 feet of water is equal to 179344.2 Pa.
(b) The 220 psi is equal to
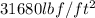
(c) The 120 torr is equal to 15998.6 Pa.
(d) The 1.0 atm to inches of glycerin is equal to 323.07 inches.
(e) The 1050 mm Hg is equal to
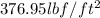
Explanation :
(a) The conversion used from feet to pascal is:
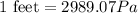
As we are given that 60 feet of water. Now we have to convert into Pa.
As,
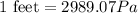
So,
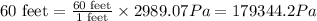
The 60 feet of water is equal to 179344.2 Pa.
(b) The conversion used from
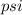 to
to
 is:
is:
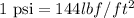
As we are given that 220 psi. Now we have to convert into
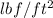 .
.
As,
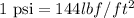
So,
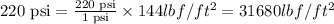
The 220 psi is equal to
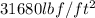
(c) The conversion used from torr to pascal is:
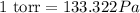
As we are given that 120 torr. Now we have to convert into Pa.
As,
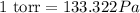
So,
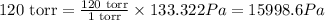
The 120 torr is equal to 15998.6 Pa.
(d) 1.0 atm to inches of glycerin
Formula used :
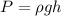
where,
P = pressure of glycerin = 1.0 atm = 101325 Pa
g = acceleration due to gravity =
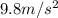
 = density of glycerin =
= density of glycerin =
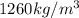
Now put all the given values in above formula, we get:
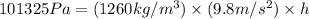
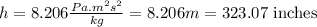
Conversion used :
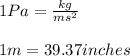
The 1.0 atm to inches of glycerin is equal to 323.07 inches.
(e) The conversion used from
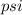 to
to
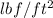 is:
is:
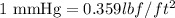
As we are given that 1050 mmHg. Now we have to convert into
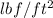 .
.
As,
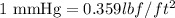
So,
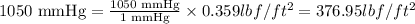
The 1050 mm Hg is equal to