Answer:
D. [H+] of the acetic acid solution is greater than that of the hydrocyanic acid solution.
Step-by-step explanation:
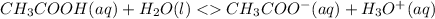
Initial 0.1M 0 0
Change -x +x +x
Equilibrium 0.1M-x +x +x
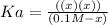
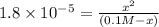
(-x is neglected) so we get
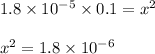
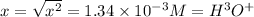 is the
is the
![[H^+ ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/qggmz3jtq6ewy98hz94dtelwv9aenue0s8.png) concnetration of acetic acid
concnetration of acetic acid
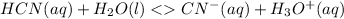
Initial 0.1M 0 0
Change -x +x +x
Equilibrium 0.1M-x +x +x
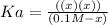
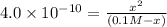
(-x is neglected) so we get
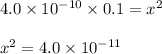
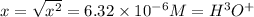 is the
is the
![[H^+ ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/qggmz3jtq6ewy98hz94dtelwv9aenue0s8.png) concnetration of Hydrocyanic acid
concnetration of Hydrocyanic acid
Thus we see here, [H+] of the acetic acid solution
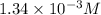 is greater than that of the hydrocyanic acid solution
is greater than that of the hydrocyanic acid solution
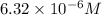
Please note∶
To find
![[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/v2s9jc0jpiravka8sb1nzyuu8dz1xobytx.png) we use formula
we use formula
![[OH^- ]= \frac {kw}{([H^+])}](https://img.qammunity.org/2020/formulas/chemistry/middle-school/3jhq4kepfdgcd9nd562xeih04dd9hg4imh.png)
The value of kw is 1.0×10^(-14)
The
![[OH^- ]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/v2s9jc0jpiravka8sb1nzyuu8dz1xobytx.png) is lesser in each solution
is lesser in each solution