Answer: endothermic
Step-by-step explanation:
Exothermic reactions are defined as the reactions in which energy of the product is lesser than the energy of the reactants. The total energy is released in the form of heat and
 for the reaction comes out to be negative.
for the reaction comes out to be negative.
Endothermic reactions are defined as the reactions in which energy of the product is greater than the energy of the reactants. The total energy is absorbed in the form of heat and
 for the reaction comes out to be positive.
for the reaction comes out to be positive.
Given :
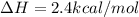
As heat change is positive, the reaction is endothermic. Energy of products is more as compared to energy of reactants and thus energy is absorbed.