Answer:
Q=36444.11 Btu
Step-by-step explanation:
Given that
Initial temperature = 60° F
Final temperature = 110° F
Specific heat of water = 0.999 Btu/lbm.R
Volume of water = 90 gallon
Mass = Volume x density
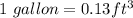
Mass ,m= 90 x 0.13 x 62.36 lbm
m=729.62 lbm
We know that sensible heat given as
Q= m Cp ΔT
Now by putting the values
Q= 729.62 x 0.999 x (110-60) Btu
Q=36444.11 Btu