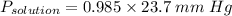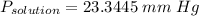Answer:
the vapor pressure of water over the solution is 23.3445 mm Hg
Step-by-step explanation:
From Raoult's law:
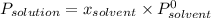
where:
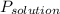 is the observed pressure above the solution
is the observed pressure above the solution
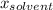 is the molar fraction of solvent (here water)
is the molar fraction of solvent (here water)
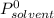 is the vapor pressure of pure solvent at a given temperature
is the vapor pressure of pure solvent at a given temperature
Moles of urea = (5 g)/(60.06 g/mol) = 0.083 mol
Moles of water = (100 g)/(18 g/mol) = 5.556 mol
Mole fraction of water = 5.556/(5.556 + 0.083) = 0.985
Therefore: