Given :
The half-life of carbon 14 is 5,730 years.
To Find :
How much would be left of an original 50- gram sample after 2, 292 years.
Solution :
We know, rate law for first order reaction is given by :
![[A] = [A_o]e^(-kt)](https://img.qammunity.org/2022/formulas/mathematics/high-school/nz7imrgeia5z57ceo39kacza4s69ps31uc.png) ...1)
...1)
Here,
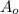 is initial amount and
is initial amount and
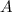 is final amount.
is final amount.
And
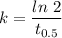
Putting value of half time in above equation, we get :
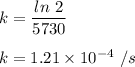
Putting value of k in equation 1) , we get :
![[A] = 50* e^{-1.21* 10^(-4)* 2292} = 37.89\ gm\\\\](https://img.qammunity.org/2022/formulas/mathematics/high-school/yf49fykkrgmfras4qz1xnzv3xfvroigh8e.png)
Hence, this is the required solution.