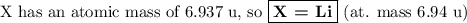Answer:
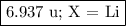
Step-by-step explanation:
1. Write the unbalanced equation
X + N₂ ⟶ X₃N
2. Balance the equation and gather all the data.
MM: 28.01
6X + N₂ ⟶ 2X₃N
m/g 1.486 1.000
3. Calculate the moles of N₂
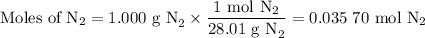
4. Calculate the moles of X
The molar ratio is 6 mol X: 1 mol N₂
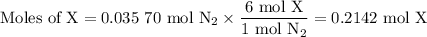
5. Calculate the molar mass of X
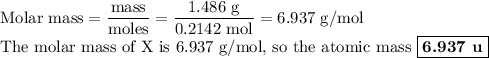
6. Identify X.