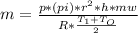Answer:
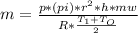
Step-by-step explanation:
The gas ideal law is
PV= nRT (equation 1)
Where:
P = pressure
R = gas constant
T = temperature
n= moles of substance
V = volume
Working with equation 1 we can get
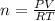
The number of moles is mass (m) / molecular weight (mw). Replacing this value in the equation we get.
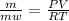 or
or
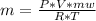 (equation 2)
(equation 2)
The cylindrical container has a constant pressure p
The volume is the volume of a cylinder this is
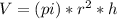
Where:
r = radius
h = height
(pi) = number pi (3.1415)
This cylinder has a radius, r and height, h so the volume is
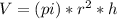
Since the temperatures has linear distribution, we can say that the temperature in the cylinder is the average between the temperature in the top and in the bottom of the cylinder. This is:
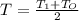
Replacing these values in the equation 2 we get:
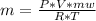 (equation 2)
(equation 2)