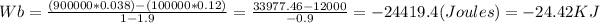Answer:
The final volume is: 0.038 m3 adn the work for the process is: -24.42 KJ
Step-by-step explanation:
The equation given for a polytropic process
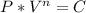 when n and C are constants. First we determine the C with the initial conditions:
when n and C are constants. First we determine the C with the initial conditions:
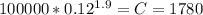 , we need to remember that 1 bar is the same to 100000 Pascals. Then we can calculate the final volume solving the equation given to:
, we need to remember that 1 bar is the same to 100000 Pascals. Then we can calculate the final volume solving the equation given to:
![Vfinal=\sqrt[n]{(C)/(Pfinal)}](https://img.qammunity.org/2020/formulas/physics/college/fbmpq43wz9w7ogwn4lk69h4yqd3jb3kml4.png) so we get:
so we get:
![Vfinal=\sqrt[1.9]{(1780)/(900000) } = 0.038(m3)](https://img.qammunity.org/2020/formulas/physics/college/14p09ck1r2m45tz0tz4kw0lzul9jqqeiyx.png) . Now using the integration for work:
. Now using the integration for work:
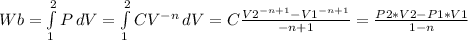 , replacing data we find the work process like:
, replacing data we find the work process like: