Answer : The given chemical reaction is:
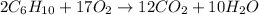
Initial 0.427 1.41 0 0
Change -2x -17x +12x +10x
Final 0.427-2x 1.41-17x 12x 10x
Explanation :
First we have to calculate the moles of
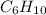 and
and

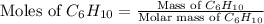
Mass of
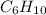 = 35 g
= 35 g
Molar mass of
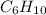 = 82 g/mol
= 82 g/mol
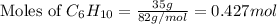
and,
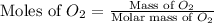
Mass of
 = 45 g
= 45 g
Molar mass of
 = 32 g/mol
= 32 g/mol
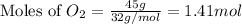
Now we have to complete the ICE table.
The given chemical reaction is:
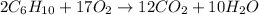
Initial 0.427 1.41 0 0
Change -2x -17x +12x +10x
Final 0.427-2x 1.41-17x 12x 10x