Answer:
435 nm
Step-by-step explanation:
Given the energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kJ/mol
⇒ energy per photon =energy per mole/Avogadros number
=
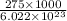
= 4.5666 x 10^(-19) J
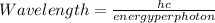
=
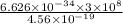
=
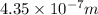
=435 nm
hence the wavelength is equal to 435 nm