Step-by-step explanation:
Relation between molarity and molar mass is as follows.
Molarity =
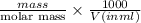
It is given that mass is 1 mg/ml which is also equal to
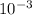 g.
g.
Molecular mass = 58 Da = 58 g/mol
Volume = 1 ml
Therefore, calculate molarity as follows.
Molarity =
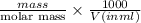
=
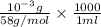
= 0.0172 molar
It is known that 1 molar equals 1000 millimolar.
So, 0.0172 molar =
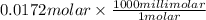
= 17.2 millimolar
Thus, we can conclude that the concentration of given solution is 17.2 millimolar.