Answer:
2790 Pa
Step-by-step explanation:
Given wavelength λ= 5μm
temperature T= 400 K
cross section of collision σ= 0.28 nm^2
molar mass = 39.9 g/mole
pressure =
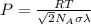
putting values we get
=
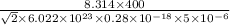
⇒P = 2790 J/m^3
the partial pressure are argon atoms expected= 2790 Pa