Answer:
The percent by mass of chromium(II) acetate in the solution is 4.42%.
Step-by-step explanation:
Molality of the chromium(II) acetate solution = 0.260 m = 0.260 mol/kg
This means that in 1 kg of solution 0.260 moles of chromium(II) acetate are present.
1000 g = 1 kg
So, in 1000 grams of solution 0.260 moles of chromium(II) acetate are present.
Then in 100 grams of solution =
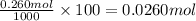
Mass of 0.0260 moles chromium(II) acetate:
= 0.0260 mol × 170 g/mol = 4.42 g
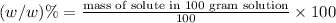
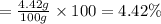
The percent by mass of chromium(II) acetate in the solution is 4.42%.