Answer : The value of activation energy for this reaction is 108.318 kJ/mol
Explanation :
The Arrhenius equation is written as:
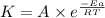
Taking logarithm on both the sides, we get:
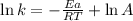 ............(1)
............(1)
where,
k = rate constant =
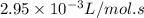
Ea = activation energy = ?
T = temperature = 435 K
R = gas constant = 8.314 J/K.mole
A = pre-exponential factor =
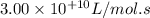
Now we have to calculate the value of rate constant by putting the given values in equation 1, we get:
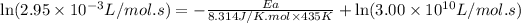
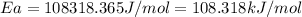
Therefore, the value of activation energy for this reaction is 108.318 kJ/mol