Answer:
The pH of the resulting solution is 12.52.
Step-by-step explanation:
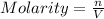
n = number of moles
V = volume of the solution in Liters
1)1 mol of HCl gives 1 mol of hydrogen ion.
![[HCl]=[H^+]=0.1 m](https://img.qammunity.org/2020/formulas/chemistry/college/hcwriqmc95rg589tpapj513d2wucn4f0fq.png)
Concentration of the hydrogen ion = 0.1 M
Volume of the solution = 10 mL = 0.010 L
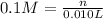
Moles of hydrogen ions = = 0.001 mol
2) 1 mol of NaOH gives 1 mol of hydroxide ion.
![[NaOH]=[OH^-]=0.2 M m](https://img.qammunity.org/2020/formulas/chemistry/college/ctxmy56hxgwzl67r7vr11ro7a78td3ypfs.png)
Concentration of the Hydroxide ions = 0.2 M
Volume of the solution ,V'= 8 mL = 0.008 L
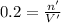
Moles of hydroxide ions ,n ' = 0.0016
1 mol of HCl neutralizes 1 mol of NaOH ,then 0.001 mol of HCl will neutralize 0.001 mol NaOH.
So left over moles of hydroxide ions in the solution will effect the pH of the solution:
Left over moles of hydroxide ions in the solution = 0.0016 mol - 0.0010 mol = 0.0006 mol
Left over concentration of hydroxide ions:
![[OH^-]'=(0.0006 mol)/(0.010 L+0.008 L)=0.0333 mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/iyu8y1jhkhuv784uf0k9fwdklpyip3rk4i.png)
![pOH=-\log[OH^-]=-\log[0.03333 M]=1.48](https://img.qammunity.org/2020/formulas/chemistry/college/dep8gtjrb3abawg66tlt88v6id7nrp4erj.png)
pH +pOH = 14
pH = 14 - 1.48 = 12.52
The pH of the resulting solution is 12.52.