Answer:
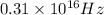 .
.
Step-by-step explanation:

 = Wavelength of radiation
= Wavelength of radiation
E= energy
Using Rydberg's Equation:
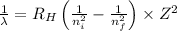
Where,
 = Wavelength of radiation = ?
= Wavelength of radiation = ?
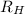 = Rydberg's Constant
= Rydberg's Constant
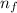 = Higher energy level = 4
= Higher energy level = 4
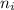 = Lower energy level = 1
= Lower energy level = 1
Z= atomic number = 1 (for hydrogen)
Putting the values, in above equation, we get
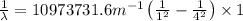
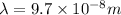
The relationship between wavelength and frequency of the wave follows the equation:
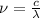
where,
 = frequency of the wave = ?
= frequency of the wave = ?
c = speed of light =
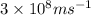
 = wavelength of the wave =
= wavelength of the wave =
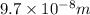
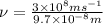
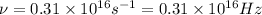
The frequency of the light emitted by a hydrogen atom during a transition of its electron from the n = 4 to the n = 1 principal energy level is
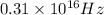 .
.