Answer:
T=340. 47 K
Step-by-step explanation:
Given that
Volume of tank =0.5
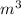
Pressure P=120 KPa
Temperature T=300 K
Added heat ,Q= 20 KJ
Given that air is treated as ideal gas and specific heat is constant.
Here tank is rigid so we can say that it is constant volume system.
We know that specific heat at constant volume for air
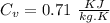
We know that for ideal gas
P V = m R T
For air R=0.287 KJ/kg.K
P V = m R T
120 x 0.5 = m x 0.287 x 300
m=0.696 kg
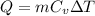
Lets take final temperature of air is T
Now by putting the values
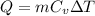
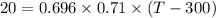
T=340. 47 K
So the final temperature of air will be 340.47 K.