Answer:
Final temperature will be 16.57°C
Step-by-step explanation:
We have given mass of nitrogen m = 6 kg
Initial temperature

Decrease in internal energy

Specific heat of nitrogen
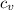 = 0.745 KJ/kg
= 0.745 KJ/kg
Let final temperature is

Change in internal energy is given by
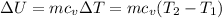
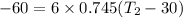
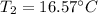
So final temperature will be 16.57°C