Step-by-step explanation:
Damkohler numbers are mainly used in chemistry. It is a dimensionless number. It denotes the timescale at which the reaction takes place with relation to the transport phenomenon.
There are two Damkohler numbers
First Damkohler number is the ratio of reaction rate to the convective mass transport rate.
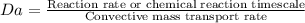
Second Damkohler number is the ratio of reaction rate to the diffusive mass transfer rate
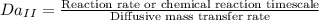
It can be seen from the equations that if the numerator is greater than the denominator then Da>1 and vice versa.
So,
When Da>1, the diffusion rate distribution is lower than the reaction rate.
When Da<1, the reaction rate is lower than the diffusion rate.