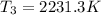Answer:
Maximum temperature of the cycle is 2231.3 K
Step-by-step explanation:
See table (values there do not assume constant specific heat) and figure attached.
Assuming ideal gas behaviour, p1*v1 = p2*v2, rearranging p2/p1 = v1/v2
Data
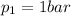
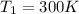
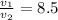 (compression ratio)
(compression ratio)
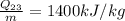 (heat addition)
(heat addition)
We can use the following relationship for air
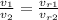
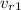 is only function of temperature and can be taken from table. In this case:
is only function of temperature and can be taken from table. In this case:
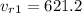
Rearranging previous equation
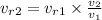
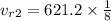
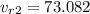
Interpolating from table
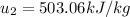
Energy balance in the process 2-3 gives
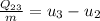
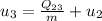
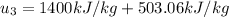
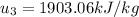
Interpolating from table