Answer:
The pressure and volume of the tank are 246.878 Kpa and 9.449 m³ respectively.
Step-by-step explanation:
Volume is constant as the tank is rigid. Take the saturation condition of water from the steam table for pressure at 127°C.
Given:
Total mass of water is 22 kg.
Mass of liquid water is 9 kg.
Temperature of water is 127°C.
From steam table at 127°C:
The pressure in the tank is 246.878 Kpa.
Specific volume of saturated water is 0.00106683 m³/kg.
Specific volume of saturated steam is 0.72721 m³/kg.
Calculation:
Step1
From steam table at 127°C:
The pressure in the tank is 246.878 Kpa.
Step2
Dryness fraction is calculated as follows:
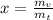
Here, dyness fraction is x, mass of vapor is
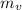 and total mass is
and total mass is
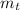 .
.
Substitute the values in the above equation as follows:
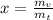
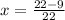
x = 0.59
Step3
Specific volume of tank is calculated as follows:
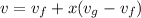
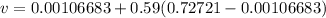
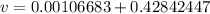
v=0.4295 m³/kg.
Step4
Volume is calculated as follows:
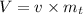
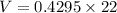
V=9.449 m³.
Thus, the pressure and volume of the tank are 246.878 Kpa and 9.449 m³ respectively.