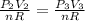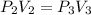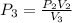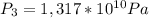Answer:
Final volumen first process
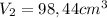
Final Pressure second process
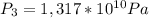
Step-by-step explanation:
Using the Ideal Gases Law yoy have for pressure:
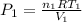
where:
P is the pressure, in Pa
n is the nuber of moles of gas
R is the universal gas constant: 8,314 J/mol K
T is the temperature in Kelvin
V is the volumen in cubic meters
Given that the amount of material is constant in the process:
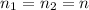
In an isobaric process the pressure is constant so:
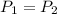
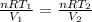
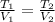
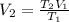
Replacing :
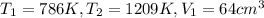
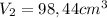
Replacing on the ideal gases formula the pressure at this piont is:
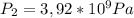
For Temperature the ideal gases formula is:
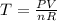
For the second process you have that
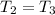 So:
So: