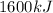Answer:
The heat transferred to water equals 1600 kJ
Step-by-step explanation:
By the conservation of energy we have
All the kinetic energy of the moving vehicle is converted into thermal energy
We know that kinetic energy of a object of mass 'm' moving with a speed of 'v' is given by
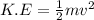
Thus
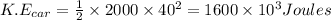
Thus the heat transferred to water equals