Answer:
The power required to cool the water is 1.11Kw.
Hence the correct option is (b).
Step-by-step explanation:
Power needed to cool down is equal to heat extract from the water.
Given:
Volume of water is 1 liter.
Initial temperature is 100C.
Final temperature is 20C.
Time is 5 minutes.
Take density of water as 100 kg/m3.
Specific heat of water is 4.186 kj/kgK.
Calculation:
Step1
Mass of the water is calculated as follows:
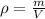
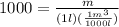
m=1kg
Step2
Amount of heat extraction is calculated as follows:
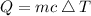
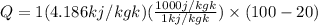
Q=334880 j.
Step3
Power to cool the water is calculated as follows:
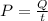
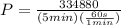
P=1116.26W
or
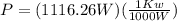
P=1.11 Kw.
Thus, the power required to cool the water is 1.11Kw.
Hence the correct option is (b).