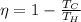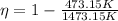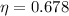Answer:
efficiency = 0.678
Step-by-step explanation:
First we have to change temperatures from °C to K (always in thermodynamics absolute temperature is used).
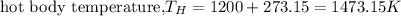
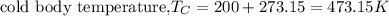
Efficiency
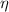 of a carnot engine can be calculated only with hot body and cold body temperature by
of a carnot engine can be calculated only with hot body and cold body temperature by