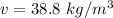Answer:
a)
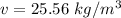
b)
Step-by-step explanation:
Given that
Pressure P = 15 MPa
Temperature = 1000 C = 1273 K
a)If assume as ideal gas
The gas constant for super heated steam R is 0.461 KJ/kg.K.
We know that ideal gas equation
P v =RT
15 x 1000 x v =0.461 x 1273
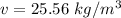
b) By using steam table
From steam table we can see that volume of super heated vapot at 15 MPa and 1273 K .