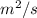Answer : The unit of (D) in metric system is
Explanation :
The given expression is:
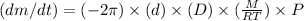
where,
m = mass
t = time
d = diameter
D = diffusion coefficient
M = molar mass
R = universal gas constant
T = temperature
P = partial pressure
In metric system,
The unit of mass is, kg
The unit of time is, s
The unit of diameter is, m
The unit of molar mass is, kg/mol
The unit of universal gas constant is,
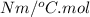
The unit of temperature is,
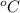
The unit of partial pressure is,
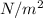
The unit of diffusion coefficient will be:
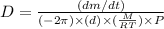
or,
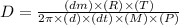
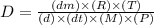
Now put all the unit in this expression, we get:
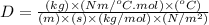
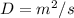
Therefore, the unit of (D) in metric system is