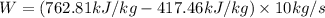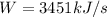Answer:
The pump work is 3451 kJ/s
Step-by-step explanation:
Pump work (W) is calculated as
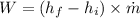
where
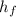 is the enthalpy of water at its final state
is the enthalpy of water at its final state
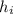 is the enthalpy of water at its initial state
is the enthalpy of water at its initial state
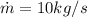 is water mass flow
is water mass flow
For liquids, properties are evaluated as saturated liquid. From the figure attached, it can be seen that
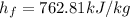
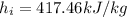
Replacing