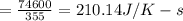Answer:
Rate of Entropy =210.14 J/K-s
Step-by-step explanation:
given data:
power delivered to input = 350 hp
power delivered to output = 250 hp
temperature of surface = 180°F
rate of entropy is given as
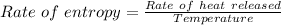
T = 180°F = 82°C = 355 K
Rate of heat = (350 - 250) hp = 100 hp = 74600 W
Rate of Entropy