Answer:
W=-280.67 KJ
Step-by-step explanation:
Given that
Initial pressure = 150 KPa
Final pressure = 600 KPa
Temperature T= 285 K
Mass m=2.4 Kg
We know that ,work in isothermal process given as
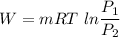
Gas constant for nitrogen gas R=0.296 KJ/kgK
Now by putting the values
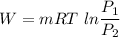
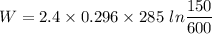
W=-280.67 KJ
Negative sign indicates that it is compression process and work is done on the gas.