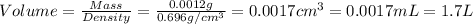Answer :
(a) The volume of ethanol liquid needed are, 2.4 L
(b) The volume of ethanol liquid needed are, 1.9 L
(c) The volume of ethanol liquid needed are, 1.6 L
(d) The volume of ethanol liquid needed are, 1.7 L
Explanation : Given,
Volume of solution = 250 mL = 0.250 L (1 l = 1000 mL)
Concentration of solution = 350 mM = 0.350 M (1 mM = 0.001 M)
First we have to calculate the moles of solution.
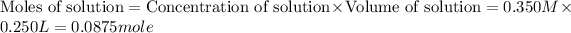
(a) For ethanol liquid :
To we have to calculate the mass of ethanol.
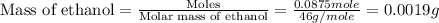
Now we have to calculate the volume of ethanol.
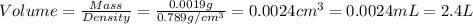
(b) For acetone liquid :
To we have to calculate the mass of acetone.
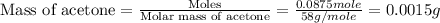
Now we have to calculate the volume of acetone.
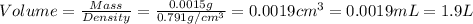
(c) For formic acid liquid :
To we have to calculate the mass of formic acid.
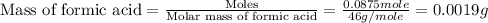
Now we have to calculate the volume of formic acid.
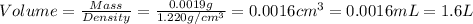
(d) For tert-Butylamine liquid :
To we have to calculate the mass of tert-Butylamine.
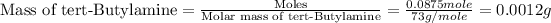
Now we have to calculate the volume of tert-Butylamine.