Answer:
M =2.33 kg
Step-by-step explanation:
given data:
mass of piston - 2kg
diameter of piston is 10 cm
height of water 30 cm
atmospheric pressure 101 kPa
water temperature = 50°C
Density of water at 50 degree celcius is 988kg/m^3
volume of cylinder is
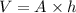
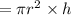
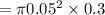
mass of available in the given container is
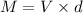
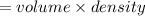

M =2.33 kg