Answer:
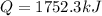
Step-by-step explanation:
Hello,
In this case, the transferred heat is defined via the first law of thermodynamics as shown below as it is about a rigid tank which does not perform any work:
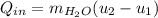
The internal energy at the first state (80°C as a vapor-liquid mixture) is computed based on its quality as follows:
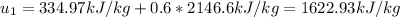
Now, the specific volume turn out into:
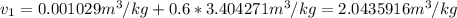
As the volume does not change due to the fact that this is about a rigid tank, we must look for a temperature at which the saturated vapor's volume matches with the previously computed volume. This turn out into a temperature of about 94.17 °C at which the internal energy of the saturated vapor is about (by interpolation):
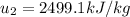
In such a way, the energy transfer by heat is:
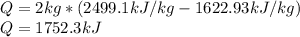
Best regards.