Answer:
m = 367.753 kg.s
Step-by-step explanation:
GIVEN DATA:
Power plant capacity W=600 MW
Plant efficiency is
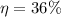
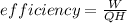
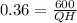
QH = 1666.6 MW
from first law of thermodynamics we hvae
QH -QR = W
Amount of heat rejection is QR = 1066.66 MW
As per given information we have 15% heat released to atmosphere
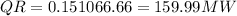
AND 85% to cooling water
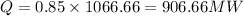
from saturated water table
at temp 150 degree c we have Hfg = 2465.4 kJ/kg
rate of cooiling water is given as = mhfg
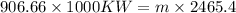
m = 367.753 kg.s
where m is rate of makeup water that is added to offset