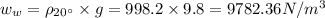Answer:
Specific weight of the pop,
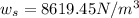
Density of the pop,
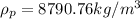
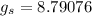
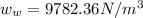
Given:
Volume of pop, V = 360 mL = 0.36 L =
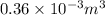
Mass of a can of pop , m = 0.369 kg
Weight of an empty can, W = 0.153 N
Solution:
Now, weight of a full can pop, W
W' = mg =
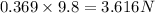
Now weight of the pop in can is given by:
w = W' - W = 3.616 - 0.513 = 3.103 N
Now,
The specific weight of the pop,
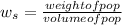
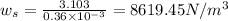
Now, density of the pop:
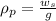
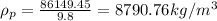
Now,
Specific gravity,
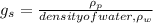
where
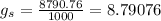
Now, for water at
 :
:
Specific density of water =
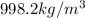
Specific gravity of water =
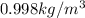
Specific weight of water at
 :
: