Answer:
percentage change in volume is 2.60%
water level rise is 4.138 mm
Step-by-step explanation:
given data
volume of water V = 500 L
temperature T1 = 20°C
temperature T2 = 80°C
vat diameter = 2 m
to find out
percentage change in volume and how much water level rise
solution
we will apply here bulk modulus equation that is ratio of change in pressure to rate of change of volume to change of pressure
and we know that is also in term of change in density also
so
E =
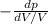 ................1
................1
And
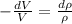 ............2
............2
here ρ is density
and we know ρ for 20°C = 998 kg/m³
and ρ for 80°C = 972 kg/m³
so from equation 2 put all value
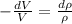
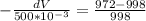
dV = 0.0130 m³
so now % change in volume will be
dV % =
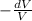 × 100
× 100
dV % =
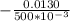 × 100
× 100
dV % = 2.60 %
so percentage change in volume is 2.60%
and
initial volume v1 =
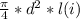 ................3
................3
final volume v2 =
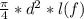 ................4
................4
now from equation 3 and 4 , subtract v1 by v2
v2 - v1 =
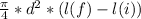
dV =
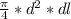
put here all value
0.0130 =
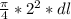
dl = 0.004138 m
so water level rise is 4.138 mm