Step-by-step explanation:
Given that,
Number density
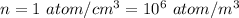
Temperature = 2.7 K
(a). We need to calculate the pressure in interstellar space
Using ideal gas equation

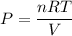
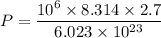
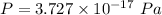

The pressure in interstellar space is
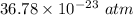
(b). We need to calculate the root-mean square speed of the atom
Using formula of rms
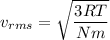
Put the value into the formula
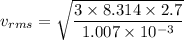
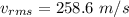
The root-mean square speed of the atom is 258.6 m/s.
(c). We need to calculate the kinetic energy
Average kinetic energy of atom
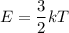
Where, k = Boltzmann constant
Put the value into the formula
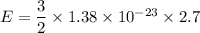
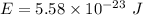
The kinetic energy stored in 1 km³ of space is
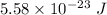 .
.
Hence, This is the required solution.