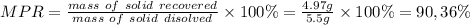Answer:
MPR=90,36%
Step-by-step explanation:
The recrystallization is a purification process where the solid to purify is dissolved in an appropriated dissolvent and then, changing the conditions the solubility changes and that solid (that was in solution before) precipitates and form crystals.
In this case, for boiling water 5.50 g of acetanilide could be dissolved and then cold the water, so the mass of crystals formed will be
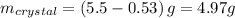
The maximum percent recovery is then