Answer:
The average atomic mass of M is 181.33 g/mol.
Step-by-step explanation:
First off we need to know the reaction that takes place. The balanced reaction of M₂S₃(s) is:
- M₂S₃(s) + O₂(g) ⇒2MO₂(s) + 3SO₂(g)
The important section for this problem is this:
**Thus, the number of moles of M in M₂S₃ is equal to the number of moles of M in 2MO₂.
The decrease in mass means that M₂S₃ reacted and produced MO₂, thus the mass of MO₂ is 3.280-0.228=3.052 g
Now let's say x is the atomic weight of M, and write the molecular weights (Mw) of those two compounds:
Mw of M₂S₃= 2x + 96 g/mol
Mw of MO₂= x + 32 g/mol
Now we determine the moles of each compound, using the formula [ moles = mass / molecular weight ]:
Moles of M₂S₃=
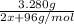
Moles of MO₂=
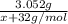
Using the equivalence marked by asterisks, we're left with (note that the second denominator is multiplied by 2 because of the reaction coefficients):
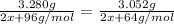
We solve for x:
![(3.280g)/(3.052g)=\frac{2x+96g/mol}{2x+64g/mol[tex]1.075*(2x+64g/mol)=2x+96g/mol\\2.150x+68.8g/mol=2x+96g/mol\\0.150x=27.2g/mol\\x=181.33g/mol]() }[/tex]
}[/tex]
Thus, the average atomic mass of M is 181.33 g/mol.