Answer:
17.1195 grams of nitric acid are produced.
Step-by-step explanation:
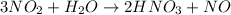
Moles of nitrogen dioxide :
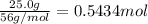
According to reaction 3 moles of nitrogen dioxides gives 2 moles of nitric acid.
Then 0.5434 moles of nitrogen dioxides will give:
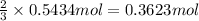 of nitric acid.
of nitric acid.
Mass of 0.3623 moles of nitric acid :
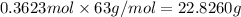
Theoretical yield = 22.8260 g
Experimental yield = ?
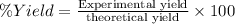
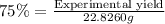
Experimental yield of nitric acid = 17.1195 g