Answer:
False
Step-by-step explanation:
Molality is one of the ways of expressing the concentration of a solution. It is defined as the number of moles of solute per kilogram of solution. It's unit is mol/kg or molal. It has a wide variety of application in estimating concentration.
To find the molality of a solution:
Molality =
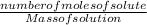
Note: a measure of the number of moles per liter of solution is called molarity.