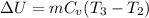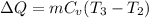Answer:
For isentropic process : Q= 0 ,
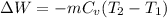
For constant volume :
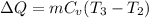 ,W=0
,W=0
Step-by-step explanation:
Given that:
System is closed
In first process ideal gas goes an isentropic expansion and then constant volume heat rejection.
We know that from first law of thermodynamic
Q = ΔU + W
For process 1-2:
Process is adiabatic it means that Q=0
So Q = ΔU + W
0 = ΔU + W
W= -ΔU
We know that internal energy for ideal gas
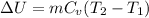
So work W
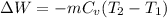
For process 2-3:
Process is constant volume so work transfer will be zero ,W=0
Q = ΔU + W
Q = ΔU + 0
Q = ΔU