Answer:
The rise in temperature of helium gas is 14.25 K.
Step-by-step explanation:
Given that
H= 4.5 km
The box is insulated ,at 4.5 km height helium gas have potential energy and when it come down then this potential energy will convert into the internal energy of helium gas.
So by heat balance
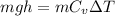
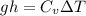
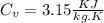 for helium gas
for helium gas
Now by putting the values
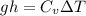
10 x 4.5 =3.15 x ΔT
ΔT=14.25 K
So the rise in temperature of helium gas is 14.25 K.