Answer : The heat energy needed would be, 6486.5125 J
Explanation :
To calculate the change in temperature, we use the equation:
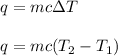
where,
q = heat needed = ?
m = mass of aluminum = 223 g
c = specific heat capacity of aluminum =
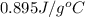
 = change in temperature
= change in temperature
 = initial temperature =
= initial temperature =
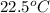
 = final temperature =
= final temperature =
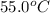
Putting values in above equation, we get:
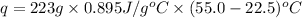
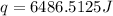
Therefore, the heat energy needed would be, 6486.5125 J